Alkaline Anion Exchange Membranes
Combinatorial Catalyst Screening
|
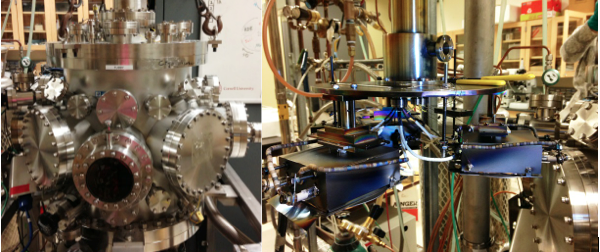
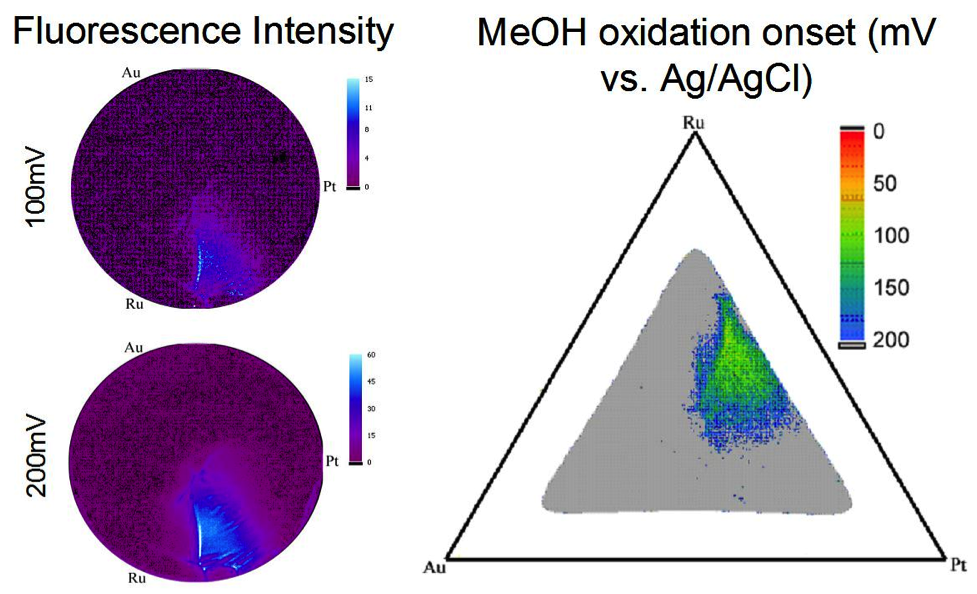
With future energy needs growing, it has become essential for alternative energy conversion technologies to be explored, one of which being fuel cells. Factors limiting further application of fuel cells are that known electrocatalysts for both the anode and cathode are expensive, not very efficient, intolerant to fuel contaminants, and unstable over time. This project focuses on the high throughput screening of possible electrocatalysts for fuel cell applications. Figure 2. (left) The sputtering chamber used to deposit the combinatorial libraries. (right) The sample holders inside the chamber that allow deposition of 3 different films at one time. Libraries can be studied using a few different methods, including a fluorescence assay, and a modified electrochemical cell. Both facilitate the study of libraries on 3” Si wafers without breaking them apart. The fluorescence assay works by the addition of indicators that fluoresce with a pH decrease. When particular catalyst areas oxidize the fuel, the local pH decreases, leading to fluorescence. The greater the fluorescence, the more active the catalyst. These areas can then be mapped onto a phase diagram to determine the composition (Figure 3). The modified electrochemical cell can be used to do cyclic voltammetry of particular areas of the library. Figure 3. The fluorescence intensity increases in the active region as more positive potentials are applied. On the right the MeOH fluorescence onset potentials are displayed on a ternary phase diagram projection. The sputtered glassy carbon can help test electrocatalysts using a different technique, rotating disk electrode voltammetry, or RDE. RDE allows for the determination of mechanistic information, enabling the differentiation between different reaction pathways. 4H+ + 2O2 + 4e– → 4H2O Although this reaction would be ideal, many catalysts will only catalyze this reaction through to an intermediate—H2O2. Not only does this reduce the number of electrons consumed at the cathode, but H2O2 can also oxidize and destroy the membrane in the fuel cell. When looking for new catalysts, then, we want to find ones that will catalyze the full reaction and will do so at potentials that maximize the voltage of the fuel cell. |
Ordered Intermetallics (Fuel Cell)
|
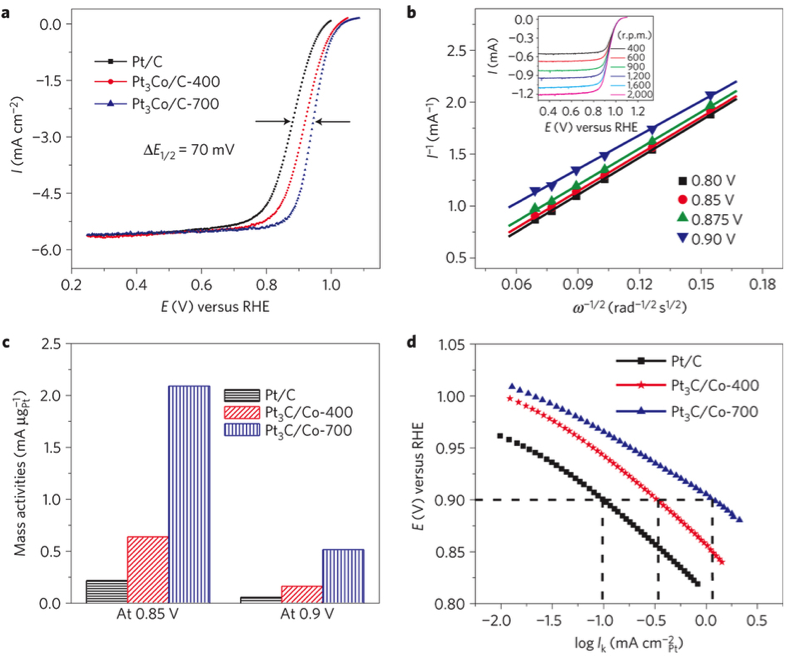
We are involved in extensive efforts to discover new materials for fuel cell applications. Ordered intermetallic compounds have been shown to exhibit remarkable electrocatalytic activity for both fuel oxidation and oxygen reduction in terms of onset potential and current density. They need to be prepared in nanoparticle form, so as to have increased surface area, which is the most effective way of increasing activity per amount of precious metals. We have prepared nanoparticles of ordered intermetallic phases by reducing metal oxides or salts using various powerful reducing agents. Our group has designed, synthesized and characterized a series of Pt/Pd-based intermetallic nanoparticles, and investigated on their structure and catalytic activity. For example, Pt3Co core-shell ordered intermetallic particles exhibited over 200% increase in mass activity compared to disordered Pt3Co alloy and commercially available Pt nanoparticles, and showed minimal loss of activity and structure after 5,000 cycles1. Figure 4. ORR polarization curves for Pt/C, Pt3Co/C (annealed at 400°C) and Pt3Co/C (prepared at 700°C) in O2-saturated 0.1 M HClO4 at room temperature, with rotation rate, 1,600 rpm and sweep rate, 5 mV s−1. b, The Koutecky–Levich plots from ORR data for Pt3Co/C-700 at different potentials. The inset in b shows the rotation-rate-dependent current–potential curves. c, Comparison of mass activities for Pt/C, Pt3Co/C-400 and Pt3Co/C-700 at 0.85 and 0.9 V. d, Comparison of specific activities (Ik). Figure 5. The chemical microstructure of the surface of the Pt-rich Pt3Co/C-700 nanoparticles was examined using electron energy loss spectroscopic (EELS). It can be seen that its structure is highly ordered with 5 nm-Pt shell, which is 2–3 atomic layers. Nanoparticle synthesis is also the final step of our combinatorial material exploration, because nanoparticles are what will be used in fuel cell stacks to maximize surface area and therefore mass activity. Citations 1. Wang, D.; Xin, H. L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D. A.; DiSalvo, F. J.; Abruña, H. D. Nature Materials 2013, 12, 81–87. |