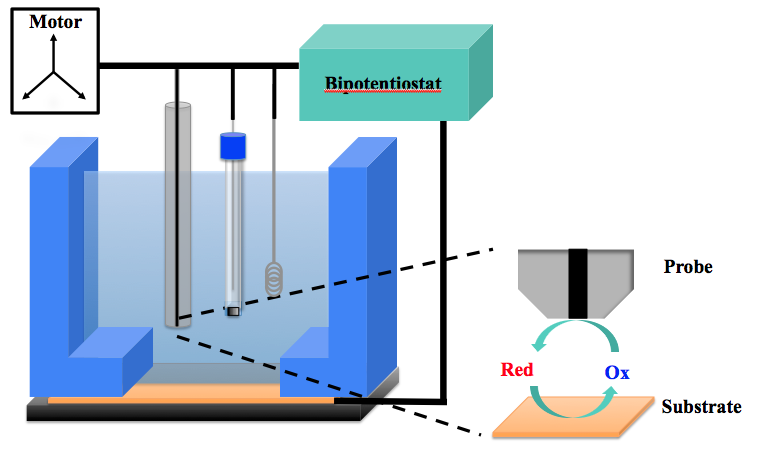
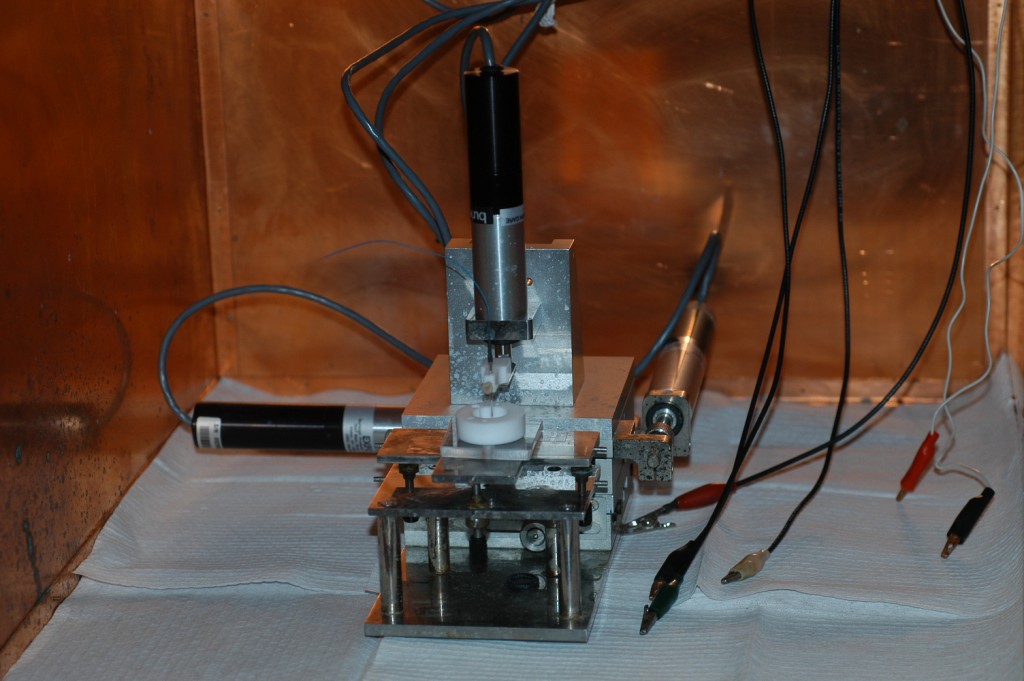
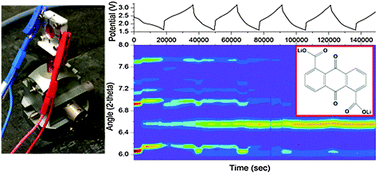
|
Electron microscopy provides tools to directly image the structure and chemistry of materials at atomic and nanometer length scales. The Abruña group uses advanced transmission electron microscopy (TEM) techniques to study materials for fuel cells and batteries.
Identical-location imaging and tomography To understand the mechanisms of fuel cell catalyst degradation, the Abruña group uses identical-location TEM, where a TEM grid is loaded with catalyst particles and used as a working electrode for electrochemical aging, allowing the morphology of Pt-Co alloy and shape-controlled Pt catalysts to be observed before and after the experiment (Fig. 1). [1,2]
Aberration-corrected Scanning TEM (STEM) imaging and electron energy-loss spectroscopy (EELS) Aberration-corrected STEM enables imaging of atomic structure at Angstrom resolution, and high beam current to enable rapid chemical imaging with EELS. The Abruña group uses these techniques to understand the detailed structure and performance of ordered intermetallic Pt3Co and dealloyed Cu3Pt fuel cell catalyst nanoparticles. [3,4]
Operando electrochemical experiments in liquid The Abruña group has developed techniques to observe electrochemical processes in operation in the TEM using a specially designed, electron-transparent electrochemical cell (Fig. 2). This has allowed the observation of charging and discharging processes in LiFePO4, a lithium ion battery cathode material, using energy-filtered TEM to track the lithium ions (Fig. 3). [5,6]
In-situ heating experiments The Abruña group has also performed in-situ heating TEM experiments to observe the dynamics of annealing and sintering in fuel cell catalyst nanoparticles. [7,8]
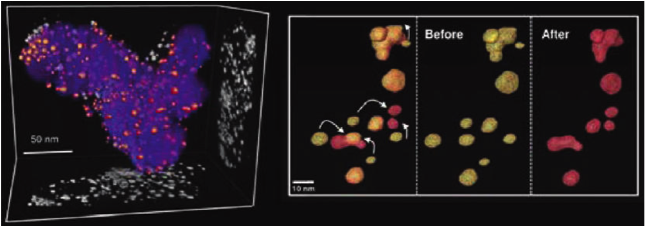
Figure 1. 3-D tomographic reconstruction of Pt-Co fuel cell catalyst before (yellow) and after (red) 30,000 electrochemical cycles between +0.6 V to +1.0 V (vs.RHE). Coalescence is revealed as the major degradation mechanism. Figure reproduced from [1].
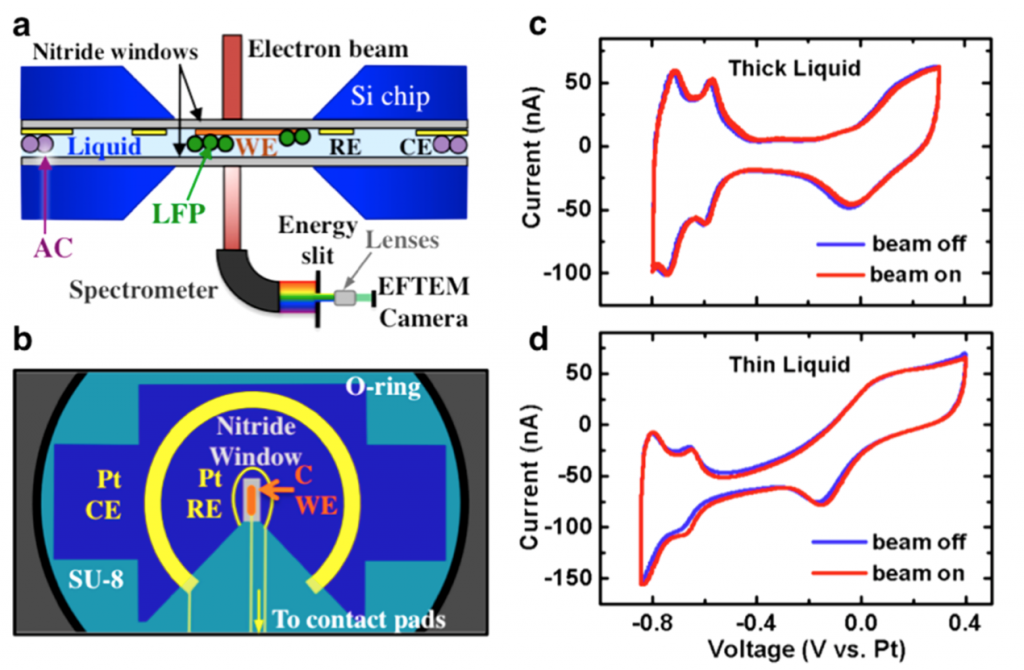
Figure 2. Schematic of the in situ electrochemistry TEM holder and electrochemical data. (a) Cross-sectional view of the holder, with silicon nitride membranes encapsulating a fluid layer. The working electrode (WE), made of carbon, lies in the viewing window, with LiFePO4 (LFP) nanoparticles deposited on top. The platinum counter electrode (CE) is coated with an excess of activated carbon (AC). In EFTEM mode, energies are selected by a slit to be imaged. (b) Schematic of the top chip, with three patterned electrodes: a carbon WE on the viewing membrane, Pt reference electrode (RE), which is not used in the battery experiment, and Pt CE. The connection leads are covered by SU8, and the contact pads to the holder do not contact the liquid so as to minimize electrochemical activity outside the viewing window. The chips exhibited electrochemical activity qualitatively similar to that of an ex situ microelectrode, as shown for the Pt cyclic voltammetry (CV) in (c) and in (d). In extremely thin liquid layers (∼150 nm), the voltammetric profile exhibits a significant ohmic drop, as seen in (d). Reproduced from [6].
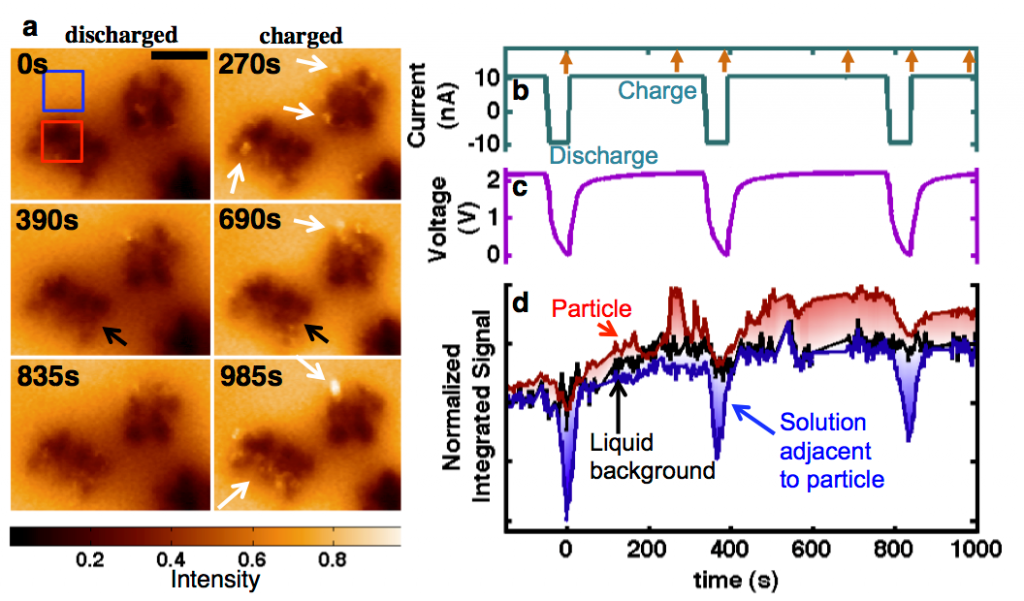
Figure 3. In situ charging and discharging of the cathode material LiFePO4 in 0.5 M Li2SO4 aqueous electrolyte. (a) The 5 eV spectroscopic EFTEM images of charging and discharging at indicated times. Scale bar is 400 nm. Bright regions are delithiated FePO4 and dark regions are LiFePO4. There are more bright regions of FePO4 at the end of charge cycles and less during the discharges. White arrows point toward “bright” charged particles, and black arrows point toward “dark” discharged particles. (b) Current profile corresponding to 10 C. Time = 0 s corresponds to the start of our study, which began on the third charge cycle (not on the first) after assembly. Arrows on the top axis indicate the times of the images shown in (a). The corresponding voltage profile is in (c), referencing the activated carbon counter electrode. (d) Integrated intensity over various regions, tracking with the voltage profile, from the regions shown by the boxes in (a). The solution becomes very dark during discharges and returns to the background level during charge. Regions of the particle are seen to light up and disappear, potentially due to delithiating and fracturing off of the particle cluster. During times when no imaging occurred, the data are linearly extrapolated, and for comparison, the intensity is brought to the same level by subtraction. Reproduced from [6].
Citations
1. Yu, Y., Xin, H. L., Hovden, R., Wang, D., Rus, E. D., Mundy, J. A., Muller, D. A. & Abruña, H. D. Three-dimensional tracking and visualization of hundreds of Pt−Co fuel cell nanocatalysts during electrochemical aging. Nano lett. 12, 4417-4423 (2012).
2. Arán-Ais, R., Yu, Y., Hovden, R., Solla-Gullón, J., Herrero, E., Feliu, J. & Abruña, H. Identical Location Transmission Electron Microscopy Imaging of Site-Selective Pt Nanocatalysts: Electrochemical Activation and Surface Disordering. J. Am. Chem. Soc. 137, 14992-14998 (2015).
3. Wang, D., Xin, H. L., Hovden, R., Wang, H., Yu, Y., Muller, D. A., DiSalvo, F. J. & Abruña, H. D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nature Materials 12, 81-87 (2013).
4. Wang, D., Yu, Y., Zhu, J., Liu, S., Muller, D. & Abruña, H. Morphology and Activity Tuning of Cu3Pt/C Ordered Intermetallic Nanoparticles by Selective Electrochemical Dealloying. Nano Letters 15, 1343-1348 (2015).
5. Holtz, M., Yu, Y., Gao, J., Abruña, H. & Muller, D. In Situ Electron Energy-Loss Spectroscopy in Liquids. Microscopy and Microanalysis 19, 1027-1035 (2013).
6. Holtz, M., Yu, Y., Gunceler, D., Gao, J., Sundararaman, R., Schwarz, K., Arias, T., Abruña, H. & Muller, D. Nanoscale Imaging of Lithium Ion Distribution During In Situ Operation of Battery Electrode and Electrolyte. Nano Letters 14, 1453-1459 (2014).
7. Chen, H., Yu, Y., Xin, H. L., Newton, K. A., Holtz, M. E., Wang, D., Muller, D. A., Abruña, H. D. & DiSalvo, F. J. Coalescence in the thermal annealing of nanoparticles: An in situ STEM study of the growth mechanisms of ordered Pt–Fe nanoparticles in a KCl matrix. Chemistry of Materials 25, 1436-1442 (2013).
8. Baumgardner, W., Yu, Y., Hovden, R., Honrao, S., Hennig, R., Abruña, H., Muller, D. & Hanrath, T. Nanoparticle Metamorphosis: An in Situ High-Temperature Transmission Electron Microscopy Study of the Structural Evolution of Heterogeneous Au:Fe2O3 Nanoparticles. ACS Nano 8, 5315-5322 (2014).
|