Computational Screening
|
We have developed computational tools to understand the electrochemical properties of molecules and materials that can be used for energy conversion technologies. Our computational cluster houses a total of 84 physical cores, which allows us to run electronic structure calculations efficiently. DFT is the method of choice and, when combined with appropriate functionals can be highly efficient in the calculation of ground state properties of electroactive compounds.Recently we have used a variety computational methods to rapidly screen new molecules for their redox potentials. These calculations have allowed us to focus on synthesis and characterization of only the most promising materials for energy storage applications, such as batteries and capacitors. Different methods allow us to calculate a variety of relevant properties. For example, to determine stability we use methods such as Nuclear Independent Chemical Shift (NICS), and Fukui indices. For excited states we have used Time Dependant DFT (TDDFT) or Zerner’s Intermediate Neglect of Differential Overlap (ZINDO). For Raman or IR spectra we have calculated force constants using computational tools available in the Gaussian package. By using these tools, we can understand better our experimental results and predict a molecule’s performance in electrochemical systems. Included below are several references where our capabilities have been used to good effect. Citations 1. Henderson, J. C.; Kiya, Y.; Hutchison, G. R.; Abruña, H. D. J. Phys. Chem. C, 2008, 112, 3989. |
Lithium-Sulfur Batteries
|
Rechargeable lithium-sulfur batteries, which use sulfur as the cathode and lithium as the anode, have been the subject of intense research in the recent past due to their high theoretical specific capacity of 1672 mAh/g and energy density of 2600 Wh/kg. However, realization of the high capacity has been precluded by insufficient control and understanding of the sulfur reduction reactions, partly due to the complexity of sulfur and polysulfide (electro)chemistry. We have studied the effects of electrolytes on the charge–discharge performance of rechargeable lithium/sulfur batteries using coin-cells. We found that the solvent in the electrolyte plays a key role in the electrochemical performance of a lithium/sulfur cell while the lithium salt has no significant effects. We also determined that carbonate-based solvents are not appropriate for lithium-sulfur batteries. Using in-situ XAS at the sulfur K-edge, we probed the sulfur reduction intermediates and products in DOL/DME, TEGDME, and EC/DEC solvents and observed a reaction between reduced sulfur species and the carbonate solvent during the course of battery discharge. We also proposed the possible reaction routes based on XAS results. Citations 1. Jie Gao, Michael A. Lowe, Yasuyuki Kiya, Héctor D. Abruña. Effects of liquid electrolytes on the charge-discharge performance of rechargeable lithium/sulfur batteries: electrochemical and in-situ X-ray absorption spectroscopic studies. J. Phys. Chem. C, 2011, 115, 25132-25137. |
Organic Cathodes
Ordered Intermetallics (Battery)
|
The Abruña group is involved in synthesis and characterization of new materials for advanced energy storage technologies. In these efforts, we make use of many fruitful collaborations through the EMC2 program at Cornell, and with other academic and industrial partners. Citations |
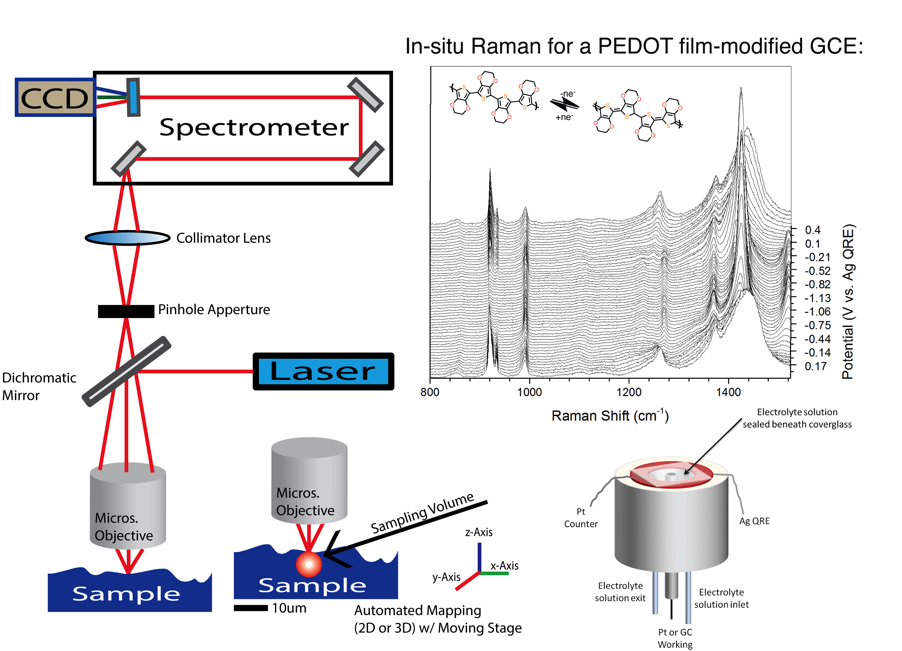
Raman Spectroscopy
Spectroelectrochemisty
|
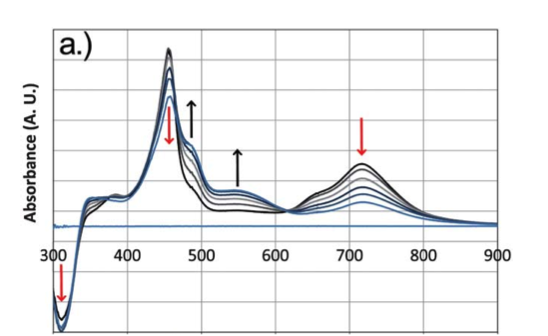
Spectroelectrochemisty is one of the techniques employed to probe battery components. Researchers design UV-Vis and/or Raman experiments to characterize compounds in solution or solid state, which includes real battery device conditions. The planning process can include designing and fabricating new cell configurations. The analysis of spectra often includes the incorporation of computational data. Figure 1. UV-Vis Spectroelectrochemistry of a small molecule in a thin layer cell. Monitoring the peak change as a function of the applied electrochemical potential. |